Produced by
From the Bench to the Bedside
The Science Behind Positive Patient Outcomes
June 2021
Faculty
Paul Stranges, PharmD, BCACP, AE-C
Clinical Assistant Professor
Department of Pharmacy Practice
College of Pharmacy
University of Illinois at Chicago
Chicago, Illinois
Introduction
The Substance Abuse and Mental Health Services Administration and American Nurses Association estimate that 10% of health care workers experience substance abuse, given the access to medications in health care institutions.1-3 One report estimates that, in 2019, 148 million medication doses were diverted by health care employees.4 This report likely underestimates the extent of diversion, given the difficulty to detect it.1,3,5 There are multiple opportunities for diversion within health care facilities: during drug procurement, preparation, administration, and waste disposal.5 “We try as much as possible to prevent diversion at all points and maintain a closed-loop accountability for medications,” said Paul Stranges, PharmD, BCACP, AE-C, a clinical assistant professor at the University of Illinois at Chicago College of Pharmacy. “We’re reliant on documentation and secure storage to maintain chain of custody for medications, but diversion can occur while drugs are in transit, in secure storage, or after dispensing and can be difficult to detect.” The issue of diversion is wide-reaching, affecting patients, practitioners, and institutions.5 In an effort to mitigate drug diversion, initiatives such as tamper-evident packaging have been put forward to enhance the chain of custody of medications. This article describes the effects of diversion on the delivery of patient care and the benefits of using tamper-evident products to mitigate them.
Diversion and Patient Safety
Diversion poses a significant threat to patient safety.2 “Diversion is first and foremost a patient safety issue,”
Dr Stranges said. “What you worry about with tampering of intravenous (IV) controlled substances (CSs) is that it goes unnoticed for a long time, and it ultimately leads to patient harm.” This can lead to numerous consequences for the patient and health care team. According to Dr Stranges, medications that are diluted or replaced with other solutions after diversion can result in inadequate pain management or anesthesia for patients, which can lead to significant harm.5 Also, there have been numerous reports of infectious disease transmission through contamination.5
“[Drug diversion] also increases the chance of introducing blood-borne pathogens or waterborne pathogens [into what] we would assume to be a sterile product,” Dr Stranges said. “Unfortunately, you hear about situations where there are outbreaks of hepatitis C or bacterial infections from contaminated drugs after something has been tampered with.”
In addition to substandard care resulting from impaired health care personnel, drug diversion poses a significant risk for practitioners trying to treat their patients and the facilities in which the care is taking place.5 “Drug diversion impacts practitioners who may be unknowingly using drugs that have been tampered with and place the Drug Enforcement Administration holder at regulatory risk,” Dr Stranges said. Any incidences of diversion at a health care facility may lead to detrimental disruptions in the delivery of patient care from audit, resulting in substantial fines and penalties as well as reputational harm.2
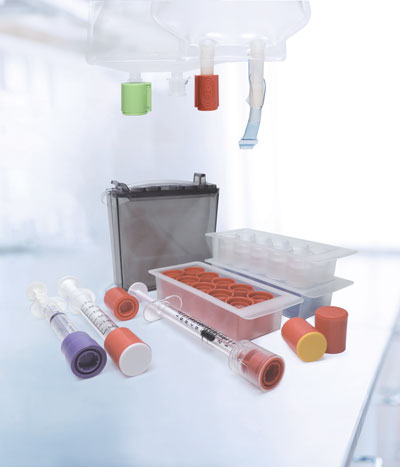
Figure. Prep-Lock Tamper Evidnet Product by IMI
Tamper-Evident Packaging and Diversion
Diversion prevention requires effective safeguards to ensure the integrity of safe medication practices. The 2013 Drug Supply Chain Security Act (DSCSA) addressed concerns regarding diversion and contamination to the drug supply chain by outlining measures to implement an electronic database by 2023, to track and trace medications and identify those that are counterfeit or diverted.6,7 Among the initiatives that have been implemented to enforce compliance with the act is the use of tamper-evident packaging. Tamper-evident packaging uses barriers or indicators that show evidence of a product being breached.8 “Tamper-evident packaging prevents somebody from accessing medications when they otherwise could have easy access,” Dr Stranges said. “It is a simple way to deter somebody from trying to divert those medications. With tamper-evident packaging, we can have confidence it’s not tampered with or adulterated.”
In 2019, 148 million medication doses were diverted by health care employees.4
Different tamper-evident packaging and technology are used at health care facilities to safeguard medications, including tape enclosures, shrink wrap, and tamper-evident caps for compounded medications.9 Tamper-evident caps for IV syringes, CADD® cassettes, and IV bags have proven to be beneficial in maintaining aseptic technique and reducing touch contamination, given their ease of use (Figure).10 For medications prepared in oral or enteral syringes, tamper-evident caps can prevent leakage and ensure that patients receive the full, intended dose.10
Tamper-evident packaging increases overall accountability in the chain of custody of the medications. The American Society of Health-System Pharmacists (ASHP) guideline on preventing diversion of CSs recommends tamper-evident packaging to ensure integrity and security of medications.2 “There had been times in which we’ve initiated investigations based off of tamper-evident products being found broken,” Dr Stranges said.
Also, USP General Chapter <797> recommends the use of tamper-evident packaging to maintain the quality of compounded sterile products.11 “All packaging should be somewhat tamper-evident,” Dr Stranges said. “Outside of diversion, it provides reassurance the product contains what is labeled and is sterile.” On a wider scope, the FDA outlines various requirements for tamper-evident packaging for certain medications prone to contamination, such as over-the-counter and 503B compounded products.8,12
Although difficult to quantify the true impact of tamper-evident packaging, Dr Stranges acknowledges the importance of using tamper-evident packaging as an effective safeguard. “Use of tamper-evident packaging gives pharmacies, health care work personnel, and patients more confidence,” he said. From the pharmacy’s perspective, “It helps us confirm what we are receiving in our pharmacy from our suppliers and what we are dispensing is what is labeled and is has not been contaminated before use.”
RFID Technology in Drug Security
The FDA has recommended tamper-evident packaging as part of a multilayered system for preventing diversion and introduction of counterfeit drugs into the drug supply chain.13 In addition, the FDA indicated that radiofrequency identification (RFID) technology may be a reliable method for reducing diversion and counterfeit products in the drug supply chain.13,14 In health care institutions, RFID is being used to track patients, medical equipment, and medications.15,16 Successful use of RFID for medication tracking has been reported in high-risk settings, such as intensive care units, and for IV medications.16 “We’re using RFID tags to automate some of our inventory management and get better insight into inventory movement,” Dr Stranges said. “It really helps our efficiency.”
A pilot program organized by the FDA is examining the use of RFID technology to develop a track-and-trace system that will comply with the DSCSA.17,18
Impact on Patient Safety
Using RFID technology to trace medications can provide granular information, such as the medication drug, dose, route, and time.16,19 Known as the 5 rights of medication administration—patient, drug, dose, route, and time— these identifiers help to reduce errors and improve patient safety.19 “You can get very detailed with RFID as far as the medication, who is in possession of it, or who is in close proximity to it, and ultimately if it’s making it to the right patient and is the right drug, dose, route, time,” Dr Stranges said. The auto-identification capabilities of RFID can link medications to interaction alerts or relevant institutional protocols, which ultimately reduce adverse events.16 The Centers for Medicare & Medicaid Services also requires medication tracking to improve patient safety.20 The tracking capabilities of RFID can be used to ensure appropriate storage of high-risk medications and quickly identify certain lots of recalled medications, as well as patients affected by recalled lots. Tracking medications during drug delivery, either by hand or in pneumatic tubes, can reduce missing medications that may result in therapy delays.21
Use of tamper-evident packaging gives pharmacies, health care work personnel, and patients more confidence.
Unlike barcode medical administration, which requires conscious thought when manually scanning an individual code, RFID is able to scan multiples codes at once in real time.15,16 “RFID takes all of the benefits of barcodes without having the limitations of needing to use barcode scanners, which have a limited area to scan medications,” Dr Stranges said. “You can use RFID to potentially scan or track movement of medications more efficiently over a larger area, and automatically.”
Diversion Mitigation
The use of RFID to track medications provides an effective approach to mitigate drug diversion.18 In fact, the ASHP guideline for preventing drug diversion recommends the use of technology and automation, such as RFID, to track medication chain of custody.2 “We have a very hands-on process for reconciling CSs. RFID is a potential way to automate some of that manual process,” Dr Stranges said. “RFID for movement of CSs is a really great area to move into.”
Integration of RFID Into Tamper-Evident Packaging
Steps are being taken to incorporate RFID technology in identifying and mitigating drug diversion. One way that this is being done is through the development of RFID-enabled tamper-evident products to secure and track sterile compounded medications.22 Products, such as tamper-evident RFID caps, can provide interoperability
between the track-and-trace requirements of the drug supply chain and hospital chain of custody.22 As part of the growing movement to expand the use of RFID in providing safe drug delivery, DoseID, an industry consortium of companies, including IMI (International Medical Industries, Inc.), was formed to establish standards for the incorporation of RFID-enabled tamper-evident products in managing the drug supply chain more effectively.22,23
Conclusion
Although the extent of drug diversion in health care is underestimated, it continues to pose a significant risk on patients, practitioners, and facilities. The use of tamper-evident packaging can provide an effective approach in identifying and mitigating diversion.6-8 Furthermore, the integration of RFID technology with tamper-evident packaging can improve patient safety, while optimizing the supply chain of custody. “If there were RFID-embedded safety caps, it would turn a 2-step process into 1,” Dr Stranges said. “We’re already adding a safety seal or cap to sterile products, and we may add an RFID tag to the package. If it’s already incorporated, it would be more efficient, and I think a good value.”
Tamper-Evident Caps with RFID Combat Diversion in Healthcare
The Staggering Cost of DiversionA recent study published in the Journal of Hospital Medicine cited the vast costs diversion poses for Health Systems. “Hospitals bear the cost of diverted drugs, internal investigations, and follow-up care for affected patients, and can...
Trends in Ensuring Safety for Home Infusion: The Case for Tamper-Evident Products
To ensure safety throughout the supply chain and in the patient’s home, providers are adopting tamper-evident products for intravenous home infusion.
To better understand the steps home infusion providers are taking to ensure safety, Becker’s Hospital Review recently spoke with Neil Colby, RPh, an infusion pharmacist and Director of Infusion Pharmacy Services for CDRx Infusion
What Are You Doing To Prevent Drug Diversion?
the record $7.75 million penalty for allegations of inappropriate pharmacy operations involving drug diversion. The Investigation by Drug Enforcement Officials uncovered irregularities concerning the distribution, dispensing, and record keeping of controlled substances…
IMI offers a comprehensive line of tamper-evident products to help protect your medications.
References
1. The Joint Commission. Quick Safety. April 2019:48. Drug diversion and impaired health care workers. April 15, 2019. Accessed April 2, 2021. https://www.jointcommission. org/-/media/tjc/newsletters/quick_safety_drug_diversion_ final2pdf.pdf
2. Brummond PW, Chen DF, Chruchill WW, et al. ASHP guidelines on preventing diversion of controlled substances. Am J Health Syst Pharm. 2017;74(5):325-348.
3. Baldisseri MR. Impaired healthcare professional. Crit Care Med. 2007;35(2 suppl):S106-S116.
4. $183M lost due to healthcare workforce diverting drugs from patient care in 2019 [news release]. Protenus; September 18, 2020. Accessed April 2, 2021. https://www.protenus. com/resources/183m-lost-due-to-healthcare-workforce-diverting-drugs-from-patient-care-in-2019
5. Berge KH, Dillon KR, Sikkink KM, et al. Diversion of drugs within health care facilities, a multiple-victim crime: patterns of diversion, scope, consequences, detection, and prevention. Mayo Clin Proc. 2012;87(7):674-682.
6. FDA. Drug Supply Chain Security Act (DSCSA). Accessed April 2, 2021. https://www.fda.gov/drugs/drug-supply-chain-integrity/drug-supply-chain-security-act-dscsa
7. FDA. Drug Supply Chain Security Act Public-Private Partnership. Accessed April 2, 2021. https://www.fda.gov/drugs/drug-supply-chain-security-act-dscsa/drug-supply-chain-security-act-public-private-partnership
8. FDA. Code of Federal Regulations Title 21, Section 211.132: tamper-evident packaging requirements for over-the-counter (OTC) human drug products. Revised April 1, 2020. Accessed April 2, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=211.132
9. Zadburke N, Shahi S, Gulecha B, et al. Recent trends and future of pharmaceutical packaging technology. J Pharm Bioallied Sci. 2013;5(2):98-110.
10. International Medical Industries. Prep-Lock™ Tamper Evident Caps. Accessed April 2, 2021. https://imiweb.com/prep-lock-tamper-evident-caps-2/
11. USP General Chapter <797> Pharmaceutical Compounding—Sterile Preparations. 2019. https://www.usp.org/sites/default/files/usp/document/our-work/compounding/proposed-revisions-gc-797.pdf
12. FDA. Current Good Manufacturing Practice—guidance for human drug compounding outsourcing facilities under Section 503B of the FD&C Act. January 2020. Accessed April 2, 2021. https://www.fda.gov/media/88905/download
13. FDA. Combating counterfeit drugs: a report of the Food and Drug Administration. February 2004. Accessed April 2, 2021. http://old.fda.gov/oc/initiatives/counterfeit/report02_04.html
14. Taylor D. RFID in the pharmaceutical industry: addressing counterfeits with technology. J Med Syst. 2014;38(11):141.
15. Martinez Perez M, Dafonte C, Gomez A. Traceability in patient healthcare through the integration of RFID technology in an ICU in a hospital. Sensors (Basel). 2018;18(5):1627.
16. Martinez Perez M, Vazquez Gonzalez G, Dafonte C. The development of an RFID solution to facilitate the traceability of patient and pharmaceutical data. Sensors (Basel). 2017;17(10):2247.
17. FDA. DSCSA Pilot Project Program. May 22, 2019. Accessed April 2, 2021. https://www.fda.gov/drugs/drug-supply-chain-security-act-dscsa/dscsa-pilot-project-program
18. MacDonald K. RFID and medical management in the hospital: a solution to an ongoing obstacle. Forbes. December 6, 2019. Accessed April 2, 2021. https://www.forbes.com/sites/forbestechcouncil/2019/12/06/rfid-and-medical-management-in-the-hospital-a-solution-to-an-ongoing-obstacle/?sh=4ef4cb63b199
19. Engels MJ, Ciarkowski SL. Nursing, pharmacy, and prescriber knowledge and perceptions of high-alert medications in a large, academic medical hospital. Hosp Pharm. 2015;50(4):287-295.
20. Centers for Medicare & Medicaid Services. Stage 2 eligible hospital and critical access hospital meaningful use core measures. Stage 2 tipsheet. August 2012. Accessed April 2, 2021. https://www.cms.gov/regulations-and-guidance/legislation/ehrincentiveprograms/downloads/stage2overview_tipsheet.pdf
21. Dorn M, Bonkowski JJ. Applications of a real-time location system in pharmacy practice. Am J Health Syst Pharm. 2014;71(24):2109-2110.
22. PPN News Staff. IMI Joins DoseID to further expand RFID interoperability in health care. Pharmacy Practice News. September 21, 2020. Accessed April 2, 2021. https://www.pharmacypracticenews.com/Industry-News/Article/09-20/IMI-Joins-DoseID-to-Further-Expand-RFID-Interoperability-in-Health-Care/59631
23. DoseID. Introducing DoseID. Accessed April 2, 2021. https://doseid.com/
Disclosure: Dr Stranges reported no relevant financial disclosures.
Disclaimer: This is one physician’s experience, results, and recommendations, so results may vary. Please see the package insert for the complete list of indications, warnings, precautions, and other important medical information.
This monograph is designed to be a summary of information. While it is detailed, it is not an exhaustive review. McMahon Publishing, International Medical Industries, Inc., and the author neither affirm nor deny the accuracy of the information contained herein. No liability will be assumed for the use of this monograph, and the absence of typographical errors is not guaranteed. Readers are strongly urged to consult any relevant primary literature.
Copyright © 2021 McMahon Publishing, 545 West 45th Street, New York, NY 10036. Printed in the USA. All rights reserved, including the right of reproduction, in whole or in part, in any form.
