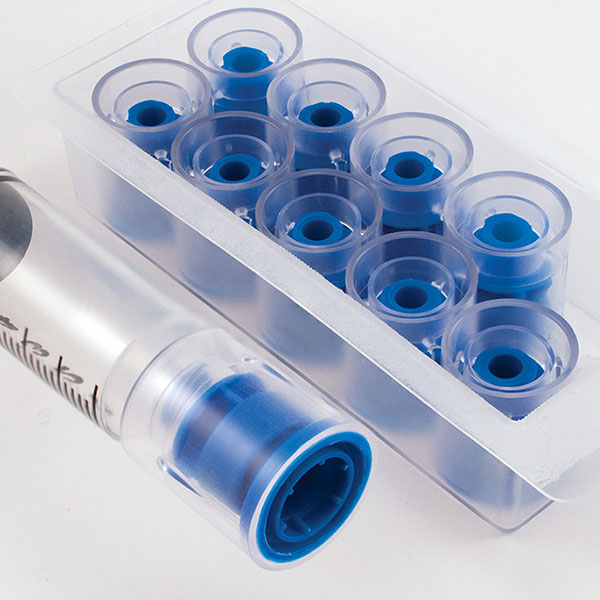
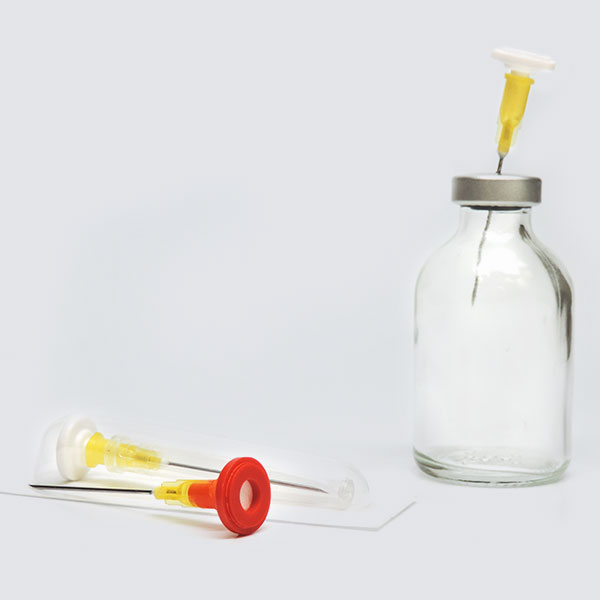
Tamper Evident Caps
For IV Syringes
For Bulk Packaging Automation Applications
Bulk Packaging for Syringe Automation Applications
International Medical Industries, Inc. is excited to announce a new packaging configuration for Tamper Evident IV Syringe Caps. This new packaging features 100 Sterile Tamper Evident Caps in a single Tyvek Pouch. Designed with high volume automated compounding operations in mind, this new configuration quickly and easily introduces Tamper Evident Caps into your syringe filling operations. The single Tyvek pouch makes for simple wipe down, expediting entry into sterile environments, Saves time a material cost over the introduction of single Tamper Evident Cap Trays, and reduces packaging consumables.

Created For Automated Syringe Filling Operations
Designed with the high-volume automated compounding opperation in mind.
Expedite Entry
Expedite Entry into Sterile Environments.
Reduce Cost
One wipe down process reduces consumed materials.
Increase Security
Deterrent to Diversion
Serves as an active deterrent to potential drug diversion and misuse.
Industry Leading
Through 50 years of service, IMI has become the standard for guarding Compounded Sterile Preparations.
DEA & USP

Prep-Lock™ Tamper Evident Caps
In Bulk Sterile Pouch
IMI is proud to have served the pharmaceutical compounding industry for 50 years by delivering high-quality American made devices that meet distinct needs. Through our partnerships with compounding professionals, we continue to advance our devices to serve the healthcare industry. The Bulk Sterile Packaging configuration is another example of how IMI’s customer-focused ethos and superior engineering capability allows us to be responsive to the need of our partners and the compounding industry. Our capabilities and highly trained teams are the reasons why we have remained the industry standard for tamper evident cap technology and why we continue to deliver customer-focused products to enhance pharmacy productivity, safety, and security.
ADVANCING THE SAFETY AND SECURITY OF YOUR DRUG PRODUCTS AND ULTIMATELY YOUR PATIENTS
Drug diversion presents innumerable risks for the healthcare industry. The installation of drug security programs continues to rise each year. Over 80% of the Hospitals now offer educational programs or guidelines on opioids. Ensuring protocol adherence and mitigating diversion temptations are central missions for these programs. Experts agree, including tamper-evident technology in your drug security program is a simple, effective way to provide an extra security level to your compounded preparations and controlled substances.
Download Instructions for Use
Click here to View & Download Instructions for Use.
Download Product Data Sheet
Color & Sleeve Options
All of IMI’s Tamper Evident Caps for IV Syringes are available in Bulk Pouch Packaging. This means you have the choice of 3 color options and two outer sleeve styles. For information about how IMI products can increase the safety and efficiency of your automated operations contact us today.






Contact Us
Have questions about what a partnership with IMI can do for your organization? What more information on a product or pricing? Fill out the form below and one of our Specialists will get back to you as soon as possible (usually within one business day) or call the number to the right.
We offer complementary evaluatory samples.
(800) 344-2554
You May Also be interested In
IMI remains at the forefront of developing new products specifically designed for the compounding industry. Our strategic partnerships with some of the biggest names in health care enable us to continusoulsy deliver the kind of high quality and high-value products that pharmacy operations demand. All IMI products are manufactured in the United States under the strictest quality standards at our FDA-registered, ISO 13485–certified facility. Browse our Comprehensive product families below, and as always our premium products are back with premium service. If you have any questions, contact us, we’re here to help!