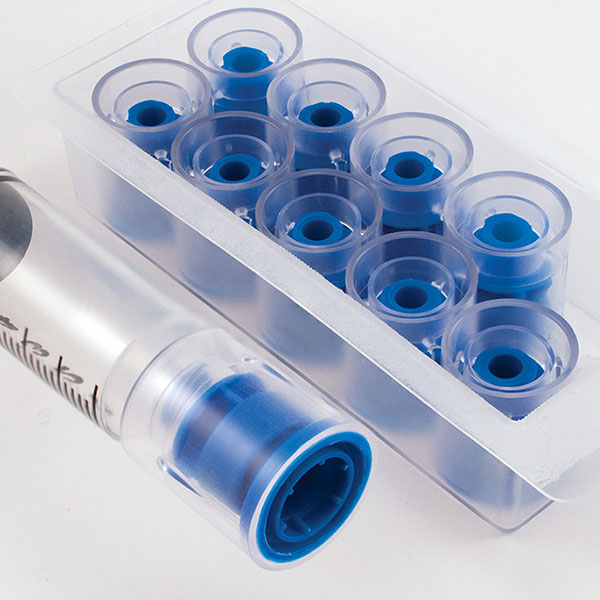
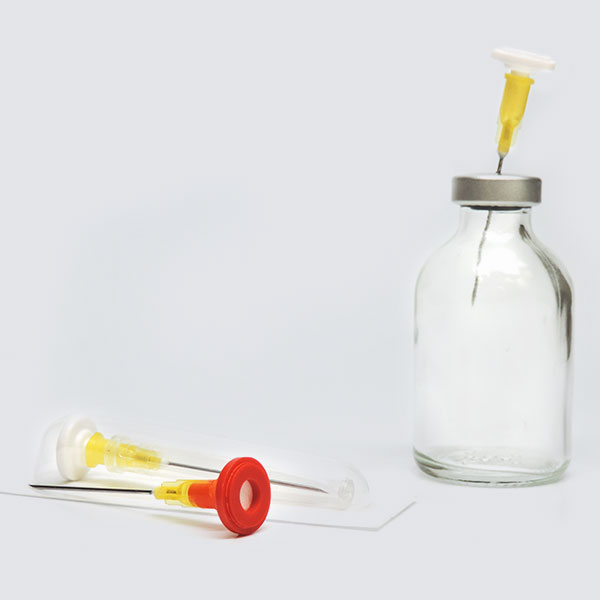
Prep-Lock™
Tamper Evident Additive Port Caps
SUPERIOR PROTECTION. SIMPLY.
Drug diversion concerns are driving the need to secure and protect a wide range of drug delivery devices.
Prep-Lock™ Tamper Evident Additive Port Cap provides remarkable protection and protocol assurance to the medication ports of compatible IV Bags, including Baxter, B.Braun, Fresenius Kabi, Douglas Medical and more.
The solid tamper-evident closure easily enhances medication safety, increases confidence in the integrity, and guards against tampering or misuse. The simply one-handed installation and considerable contribution to the integrity of IV Medications have made these caps a favorite of pharmacists everywhere.

Prep-Lock Tamper Evident Additive Port Caps
Compatible With Baxter Viaflex®, Intravia®, Aviva®, and All-In-One E.V.A.®

Download Product Information
Compatible With B. Braun PAB® & B. Braun E3®

Download Product Information
Compatible With F. Kabi FreeFlex® & Douglas Medical EcoFlx™

Download Product Information
Compatible With B. Braun Excel®

Download Product Information
Compatible With ICU Medical LifeCare®

Download Product Information
Strengthen <797> Compliance
Ensure the integrity of your compounds with USP recommended security.
Enhance Drug Security
Provide a physical barrier to the opportunity for misuse and diversion.
Simplicity & Convenience
Simple one-handed installation is a superior solution over sticky foil seals.
Deterrent to Diversion
Serves as an active deterrent to potential drug diversion and misuse.
Industry Leading
Through 50 years of service, IMI has become the standard for guarding Compounded Sterile Preparations.
DEA & USP
Tamper evident protocols may help your pharmacy comply with DEA and USP guidelines.
PROTECTION FOR YOUR IV BAG PREPARATIONS
Compounded sterile preparations (CSPs) are at their greatest risk when they leave the custody of your pharmacy. The Prep-Lock Tamper Evident Additive Port Cap greatly reduces the risk of your CSP being compromised either accidentally or intentionally. It ensures that product integrity is maintained from the time it leaves the sterile hood until it is administered by an authorized clinician. Pharmacist also greatly appreciate the additional visual indication of protocol adherence.
QUALITY AND SECURITY
The Simple, one-handed installation is pharmacists’ preferred method to providing tamper evident protection to IV Bag Preparations. The enclosed hinge and hidden lock design ensures that once the Cap is applied to the medication port, it cannot be removed without damaging the bag. Help ensure that your preparations arrive to patients without compromise.
Evaluation Samples Available
If you have questions regarding what a partnership with IMI could do for your organization, or you would like more information on a product, pricing, or requesting free samples please fill out the form below. One of our specialists will get back to you as soon as possible (usually within one business day).
Tamper-Evident Caps with RFID Combat Diversion in Healthcare
The Staggering Cost of DiversionA recent study published in the Journal of Hospital Medicine cited the vast costs diversion poses for Health Systems. “Hospitals bear the cost of diverted drugs, internal investigations, and follow-up care for affected patients, and can...
Trends in Ensuring Safety for Home Infusion: The Case for Tamper-Evident Products
To ensure safety throughout the supply chain and in the patient’s home, providers are adopting tamper-evident products for intravenous home infusion.
To better understand the steps home infusion providers are taking to ensure safety, Becker’s Hospital Review recently spoke with Neil Colby, RPh, an infusion pharmacist and Director of Infusion Pharmacy Services for CDRx Infusion
Ensuring Medication Safety with Tamper Evident Products
The Substance Abuse and Mental Health Services Administration and American Nurses Association estimate that 10% of health care workers experience substance abuse, given the access to medications in health care institutions. One report estimates that, in 2019, 148 million medication doses were diverted by health care employees.
You May Also Be Interested In
IMI remains at the forefront of developing new products specifically designed for the compounding industry. Our strategic partnerships with some of the biggest names in health care enable us to continusoulsy deliver the kind of high quality and high-value products that pharmacy operations demand. All IMI products are manufactured in the United States under the strictest quality standards at our FDA-registered, ISO 13485–certified facility. Browse our Comprehensive product families below, and as always our premium products are back with premium service. If you have any questions, contact us, we’re here to help!