Bryan McGurn, Sr. VP of Sales & Marketing Discusses IMI’s Impact In The Healthcare Domain
Evaluating IMI’s contributions to healthcare systems and specialty pharmacies alike through highly valued partnerships, advocating for customers’ voices, and adapting to new standards.
Released August 2022
Q: Diversion poses a significant risk to the healthcare systems, what role does IMI play in aiding facilities to combat this costly problem?
IMI has had a long-standing focus on developing products that significantly deter unauthorized access to the medication supply chain. Our Prep-Lock™ tamper-evident product family protects a variety of drug containers from potential misuse by offering both visual and physical barriers to those seeking an easy avenue to divert drugs and has become a valuable staple in any system’s overall drug security protocols. Our commitment to this cause does not end with our products. IMI is an active supporter of advocacy groups such as the International Health Facility Diversion Association (IHFDA) and the National Association of Drug Diversion Investigators (NADDI).
Q: Are tamper-evident products a requirement?
Ever since the intentional tampering of a commercial drug product, which led to patient deaths in the early 1980s, drug manufacturers and government legislators have introduced strong measures to mitigate such recurrences. It is no surprise that when IMI introduced its tamper-evident cap for I.V. syringes shortly thereafter, the product exploded in popularity within the drug compounding community. Today, more pressure from hospital risk management and strong recommendations from accreditation associations have brought about the further adoption of these products to improve patient safety.
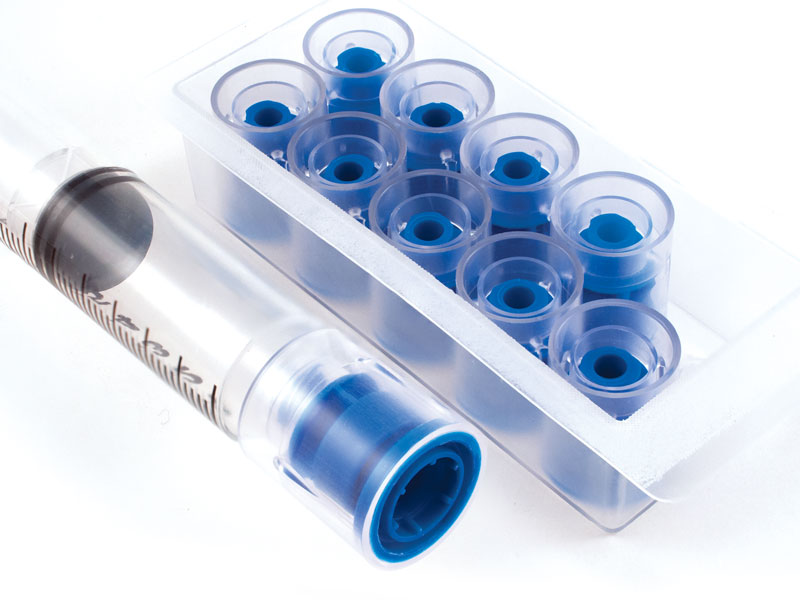
Q: How do IMI Products impact (contribute to) patient safety?
It becomes very obvious when you consider the total impact of a compromised drug. First, a drug that has been illicitly siphoned from its container may rob a patient of their intended dose. In the case of pain medication, this can substantially reduce the patient’s well-being. But the concern goes beyond that. Once that same container has been opened prior to its intended use, the chances of introducing contamination to the drug increase exponentially. This can lead to patient infection and other complications. These two reasons alone are enough to justify the use of tamper-evident products. When you consider the total cost involved in the aftermath of these events, it becomes very clear that these products are an essential component of any drug security program.
Q: IMI Tamper Evident products seem to be very popular within the outsource Compounding space. What solutions does IMI offer for health systems and specialty pharmacies?
The reason that IMI tamper-evident products are used by over 80% of the 503b Compounders should be the same reason why any compounding operation would use IMI products…Drug security and drug integrity. Compounded drug preparations are always in transit once they leave the pharmacy. The only way to ensure this medication reaches the intended patient without mishap or compromise is through the use of tamper-evident products. IMI makes it very easy to incorporate our products as part of the pharmacy workflow. We offer bulk entry packaging options to increase efficiency and reduce cost. Our products are developed by listening to the voice of the compounding pharmacist and delivering solutions to their concerns.
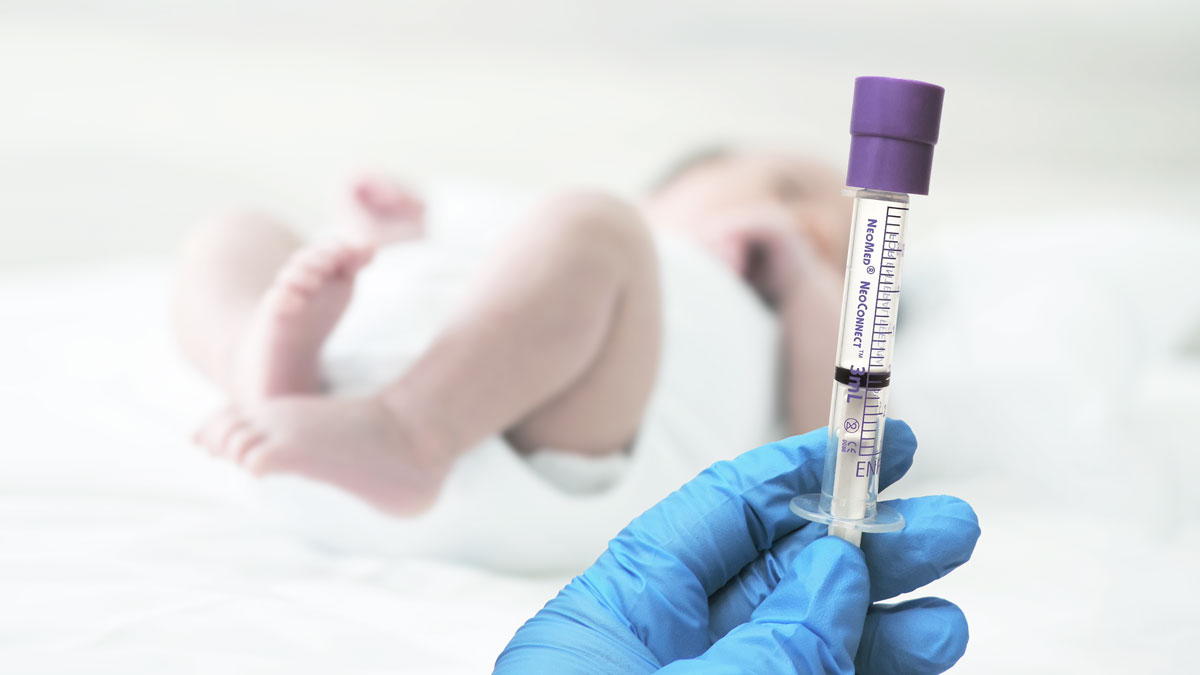
Q: The Industry is adopting a new device connector standard (ENFit) for Enteral Medications. What is IMI’s role in the transition?
IMI remains in front of the changing standards impacting the healthcare system. The ENFit connector standard is no exception. We offer the first and only tamper-evident cap product to protect syringes that comply with this connector standard. IMI is an active member of the Global Enteral Device Supplier Association (GEDSA). The organization drives the adoption of new connector standards designed to mitigate potentially harmful misconnections between various drug delivery lines, such as ENFit and the upcoming NRFit standard.
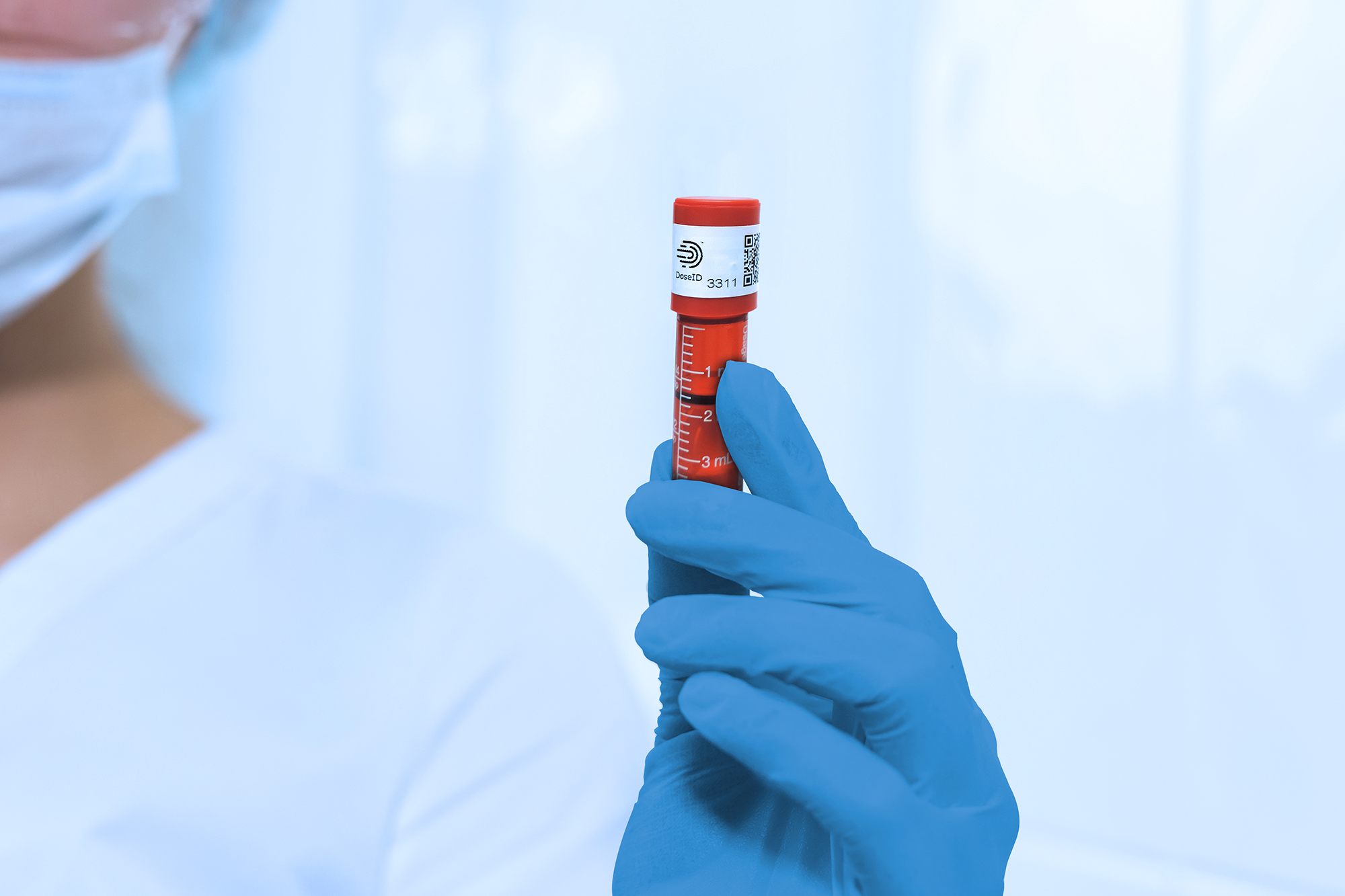
Q: Technology in the healthcare space is rapidly evolving. How is IMI innovating with the times?
IMI recognizes that it is not enough to deliver high-quality, cost-effective products. That’s why we continually innovate to address the changing technological landscape. Our new tamper-evident products with RFID are a prime example. IMI, in partnership with DoseID, is paving the way to meet the growing inventory control challenges within health systems. Our tamper-evident caps with RFID work seamlessly with DoseID-compatible inventory control systems. We make it easy to add RFID to your syringe products by doing what you should already be doing…applying an IMI tamper evident cap. There is no need to buy secondary RFID labels, installation equipment, or develop new processes or workflows. Simply install our tamper evident cap with the incorporated DoseID RFID tag.
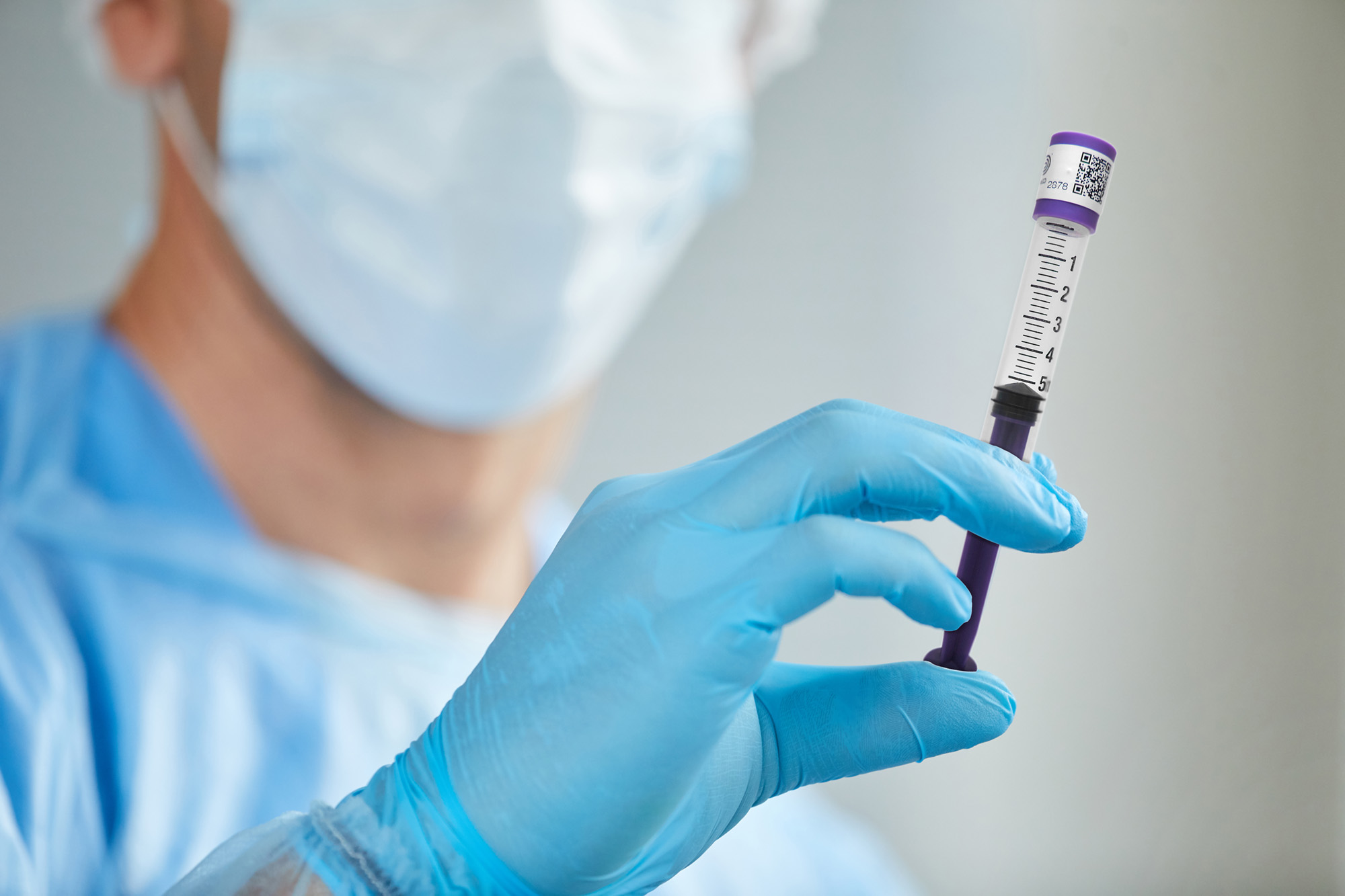
Q: How are IMI sterile compounding products different from others in the market?
Many of the topics we have discussed here exemplify IMI’s leadership position in secure drug delivery devices. Our pursuit of ongoing product innovation combined with attention to our customers has been a key to our success. In a day and age of supply chain disruptions and unstable international sources, IMI is proud to offer products that are “Made in USA.” We believe that these attributes make us uniquely qualified to be the reliable partner to pharmacists and compounding operations throughout America.
You Might Also Be Interested In Reading:
We offer complimentary evaluation samples.
Complete the form below to request yours now.
Have questions about what a partnership with IMI can do for your organization? What more information on a product or pricing? Fill out the form below and one of our specialists will get back to you as soon as possible (usually within one business day).
