The Cap That Protects Children
The cap that protects children – Children’s Hospital of Alabama bolsters secure drug delivery with the integration of IMI’s Tamper-Evident Caps for Enfit Syringes.
Pediatric Pharmacy Director ~ Children’s Hospital of Alabama
Released December 21, 2021
Intended Outcome
The theft of controlled substances or other medications meant for the patient can occur at various points in the supply chain. The installation of drug security methods at healthcare facilities continues to increase each year because of the rise and risk of drug diversion. Mitigating diversion is a priority for every health provider, and ensuring patient safety is the goal of every drug diversion method.
This case study examines why one of the simplest drug diversion solutions is also one of the most successful. Tamper evident caps are an effective tool in addressing drug diversion. Specifically, this analysis details why this product works for Children’s Hospital of Alabama (Children’s).
The success story became evident when Children’s converted to the ENFit Connector Standard to reduce enteral tubing misconnections. This conversion opened the opportunity for pharmacy leadership to purchase and prioritize tamper evident caps for the ENFit syringes, adding additional protection to the medication and Children’s.

Background
Children’s has a progressive pharmacy department that utilizes technology and clinical knowledge to provide care for a diverse patient population. It’s lead by Julie Lasseigne, pediatric pharmacy director for Children’s, who views the health and safety of children as her foremost responsibility. Lasseigne is known for solving problems and working collaboratively. She looks at this issue with zero tolerance and takes a 360 approach to combat it.
“Drug diversion is about understanding the behavior, making it difficult to access the drug, and implementing deterrents,” said Lasseigne. “I’m continually aware that compounded preparations are at their greatest risk for diversion when they leave the custody of our pharmacy.”
This 14-year-employee knows the pharmaceutical needs of Children’s. And the hospital needed tamper evident caps for their pharmaceutical security program.
“If the employee is having a bad day and feel like they need the drug, we want them to see that cap and think that it’s not worth taking it. The cap signals not to make that bad choice because you’ll get caught,” said Lasseigne.
Challenges
Drug diversion presents numerous safety and reputational risks for a hospital. Financially, hospitals bear the cost of diverted drugs, internal investigations, and follow-up care for affected patients. The government can fine the facility in excess of $4 million for inadequate safeguards. By their daily access to controlled substances, it is estimated that 15% of pharmacists, 10% of nurses, and 8% of physicians are challenged with alcohol and/or drug dependency.
The challenge that Children’s addresses every day and remains ever-vigilant is preventing the risk of inadequate dose or contamination by a clinician under the influence. The protection and safety of children involve employing multiple anti-diversion methods.
Conversion At Children’s
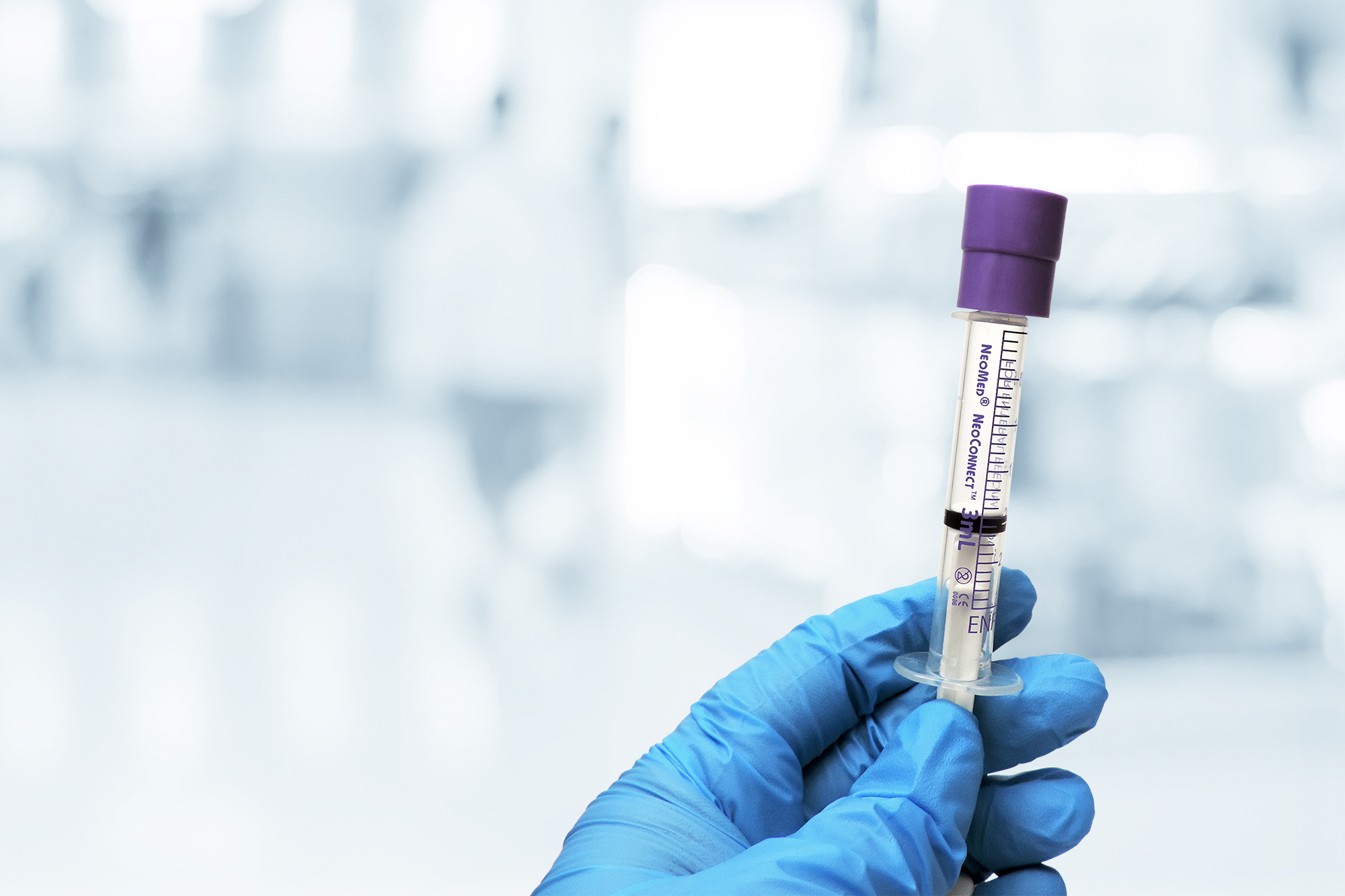
Adhering to the recommendations from Global Enteral Device Supplier Association (GEDSA), Children’s converted to the safer ISO 80369-3 compliant connectors, commonly called ENFit®, in the spring of 2021. ENFit gives every enteral delivery set, extension set, syringe, and feeding tube a specific connector unique to enteral feeding, reducing the likelihood of misconnections and accidental disconnection.
The use of slip tip syringes is no longer ISO compliant for device applications like enteral feeding. Therefore, the adoption of a new standard prompted the move to tamper evident caps for ENFit syringes.
“We met our conversion timeline as GEDSA’s manufacturers ended production of legacy feeding tubes and cross-application connectors on July 1,” said Lasseigne. “Our team also took this time to strengthen our diversion efforts.”
Supplier Decision
Lasseigne heads a multidisciplinary committee at Children’s to deploy multifaceted security programs. From high-tech systems to educational programs, Children’s continues to seek vendors to stop drug diversion.
Children’s decision to go with International Medical Industries (IMI) for tamper evident caps for ENFit syringes was an obvious decision. IMI, with its Prep-Lock™ Tamper Evident Cap Line, had specifically designed a product for ENFit.
IMI also was a reliable vendor in good standing with Children’s since 2015. They also had a proven reputation in the market with more than 85 percent of the top 503B compounding facilities trusting IMI to secure their preparations.
Beyond the strong purchasing relationship with IMI, Children’s chose their tamper evident caps because they exceeded expectations with the following areas of importance.
Safety & Integrity
Prep-Lock Tamper Evident Caps greatly reduce the risk of Children’s being compromised either accidentally or intentionally. The ENFit syringe cap possesses built-in tamper-evident technology. Compatible with low dose and standard ENFit syringes, IMI provides an additional level of security and integrity from when the product leaves the pharmacy until administered by an authorized clinician.
Once tamper evident caps are installed, they cannot be removed without evidence of potential tampering or contamination, ensuring the integrity of syringe contents. Guarding ENFit syringes with Prep-Lock tamper evident caps enhances medication safety protocols and improves patient safety.
“Product integrity is paramount when it involves medication for children,” said Lasseigne. “Tamper evident caps advance our drug diversion philosophy and enhances patient care.”
Easy & Effective
Prep-Lock products are a trustworthy, high-quality solution. They prevent leakage and ensure that patients receive the full, intended dose. Easy installation and prompt product availability make incorporation of Tamper Evident caps practically effortless.
A simple twist of the wrist equips syringes with a physical barrier to diversion, misuse, and tampering, whether intentional or unintentional, raising confidence in the integrity of medications for both pharmacist and clinicians.
“IMI delivers a product that is easy to use for my team and cost-efficient with the new GEDSA standard,” said Lasseigne. “Tamper evident caps also prove that a simple method can have a big impact to eliminate diversion at Children’s.”
Detection & Deterrence
IMI provides Children’s with clear visibility into a probable incident. A detached or missing outer sleeve indicates that a potential comprise has occurred, initiating an audit and analysis on the situation.
“When the out of the ordinary happens with controlled substances, we question why. Caps are an active deterrent to diversion and allow us to see that a syringe has been tampered with, making them an essential component to our security program.”
Standardization & Compliance
USP provides standards for the sterile preparation of pharmaceutical compounding to help ensure patient benefit and reduce risks such as contamination, infection, or incorrect dosing. Although not technically required by DEA, tamper evident caps strengthen and supplement DEA and USP guidelines. And the technology has become an industry model for securing syringes.
Incorporating the IMI caps into in-house procedures standardizes all the syringes throughoutvChildren’s, providing consistent care and streamlining processes. IMI products supply needed controls for medication integrity, and dosing accuracy.
“Consistency within pharmacy operations is always the goal, and deploying ways to reduce diversion with pediatric doses helps us achieve that goal.”
Results
Tamper evident technology in a drug security program is a simple, effective way to provide additional security to compounded preparations and controlled substances. Prep-Lock™ Tamper Evident Caps from IMI reduces the risk of Children’s being compromised either accidentally or intentionally. The standard of care is elevated with a product that guards the ENFit syringes.
“Caps for ENFit syringes are a low-cost solution for a high-confidence program at Children’s. For us, it is as simple as we like IMI, and the product works,” said Lasseigne.
Conclusion
Drug diversion concerns are driving the need to secure and protect syringe contents. Prep-Lock tamper evident caps provide an active deterrent to diversion and misuse at Children’s. The caps also help ensure that medications arrive at patients uncompromised and make a clear statement about how seriously Children’s takes drug security.
“Eliminating the opportunity to divert protects our patients and our hospital’s reputation,” said Lasseigne. “The team at IMI designed the caps for simplicity and protection. They deliver on both.”
You Might Also Be Interested In Reading:
We offer complimentary evaluation samples.
Complete the form below to request yours now.
Have questions about what a partnership with IMI can do for your organization? What more information on a product or pricing? Fill out the form below and one of our specialists will get back to you as soon as possible (usually within one business day).
